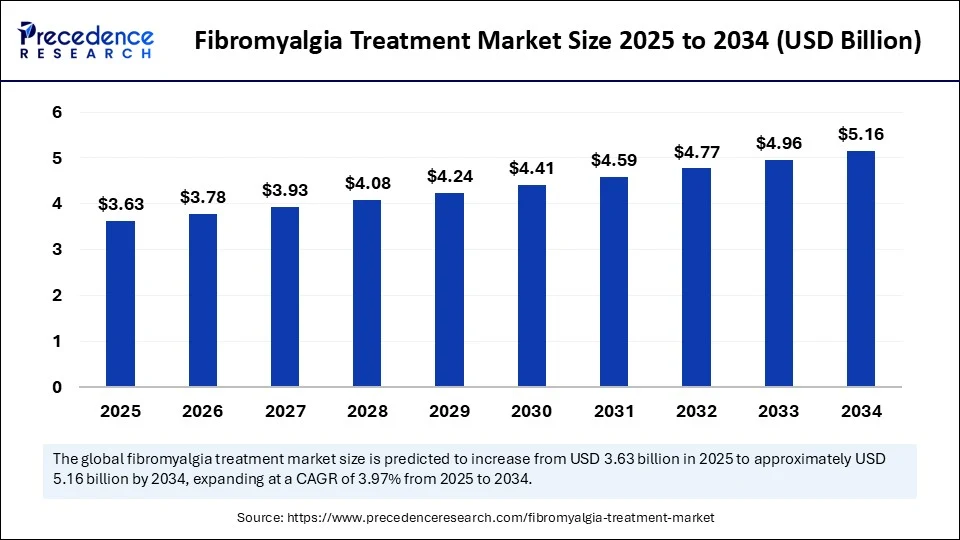
The global fibromyalgia treatment market is on a steady growth trajectory, expanding from USD 3.63 billion in 2025 to USD 5.16 billion by 2034, registering a compound annual growth rate (CAGR) of 3.97% over the forecast period. According to Precedence Research, this growth is fueled by the increasing prevalence of fibromyalgia, the rising adoption of biologics and combination therapies, and the integration of artificial intelligence (AI) in diagnostics and treatment optimization.
Fibromyalgia, a chronic pain disorder characterized by widespread musculoskeletal pain, fatigue, sleep disturbance, and cognitive difficulties, is now recognized as a major public health challenge affecting up to 8% of the global population. Growing awareness, insurance coverage, and regulatory support for innovative drugs are further expanding the treatment landscape.
Quick Insights: Fibromyalgia Treatment Market at a Glance
-
Market size (2024): USD 3.49 billion
-
Forecast size (2034): USD 5.16 billion
-
CAGR (2025–2034): 3.97%
-
Dominating Region (2024): North America (54% share)
-
Fastest-Growing Region: Asia Pacific
-
Top Treatment Type (2024): Pharmacologic therapies (55% share)
-
Fastest-Growing Treatment Segment: Digital therapeutics
-
Top Pharmacologic Class (2024): Anticonvulsants / α2δ ligands
-
Fastest-Growing Pharmacologic Class: Analgesics / NSAIDs
-
U.S. Market Outlook: Expanding from USD 1.32 billion in 2024 to USD 1.98 billion by 2034 at a CAGR of 4.14%
This Report is Readily Available for Immediate Delivery | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/6611
How is AI Shaping the Future of Fibromyalgia Treatment?
Artificial intelligence is emerging as a game-changer in fibromyalgia treatment. From drug discovery acceleration to precision diagnostics, AI is enhancing treatment pathways by:
-
Identifying potential drug candidates from vast datasets.
-
Improving diagnostic accuracy by detecting objective biomarkers.
-
Personalizing treatment plans based on genetics, lifestyle, and patient history.
-
Optimizing clinical trial design and reducing failure rates.
Expert Perspective
“Fibromyalgia has long been one of the most challenging chronic pain syndromes to diagnose and treat effectively. The integration of AI and digital therapeutics is not only enhancing accuracy but also transforming treatment into a more personalized, patient-centric journey. As pharmaceutical innovation converges with digital health, we expect a significant leap in treatment outcomes and quality of life for patients globally.”
– Dr. Aditi Rao, Principal Consultant, Precedence Research
Market Revenue Breakdowns & Coverage
| Report Coverage | Details |
|---|---|
| Market Size by 2034 | USD 5.16 Billion |
| Market Size in 2025 | USD 3.63 Billion |
| Market Growth Rate (2025–2034) | CAGR 3.97% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Segments Covered | Treatment Type, Pharmacologic Class, Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
Regional Insights
North America: The Market Leader
North America held a commanding 54% share of the global market in 2024, driven by:
-
High prevalence of fibromyalgia in the U.S. and Canada.
-
Widespread insurance coverage and access to advanced therapies.
-
Active regulatory support for innovative treatments.
-
Presence of leading pharmaceutical companies and research hubs.
The U.S. alone is projected to grow from USD 1.32 billion (2024) to USD 1.98 billion (2034), underpinned by the approval of FDA-sanctioned drugs such as pregabalin (Lyrica), duloxetine (Cymbalta), and milnacipran (Savella).
Asia Pacific: Fastest-Growing Region
Asia Pacific is set to register the highest CAGR during 2025–2034. Growth drivers include:
-
Rising incidence of fibromyalgia linked to stress and lifestyle changes.
-
Expanding healthcare infrastructure and reimbursement policies.
-
Increasing investments in research and telemedicine adoption.
-
Surge in geriatric and female populations — both more prone to fibromyalgia.
Emerging economies such as China, India, and Japan are spearheading innovation, integrating digital health solutions with conventional treatment pathways.
Read Also: Advanced Technologies in Marine Drug Development Market Size, Report by 2034
Where Are the Biggest Opportunities, and What Trends Will Shape the Market?
How Is AI Revolutionizing Fibromyalgia Diagnosis and Treatment?
Artificial intelligence is set to redefine chronic pain management by:
-
Accelerating drug discovery through advanced data analytics
-
Revolutionizing fibromyalgia diagnosis with the identification of objective biomarkers
-
Personalizing treatment strategies based on patient history, genetics, and lifestyle
-
Optimizing clinical trials and therapeutic outcomes
AI-powered platforms can assess extensive patient datasets to recommend optimal exercise regimens, medications, and pain management protocols, ushering in a new era of personalized care.
How Are Combination Therapies and Digital Solutions Enhancing Patient Outcomes?
-
Combination Therapies: Integration of biologics, pharmacologic agents, and non-pharmacologic approaches improves efficacy and expands treatment options.
-
Digital Therapeutics: Apps and wearable devices support symptom monitoring, remote engagement, and individualized care—enabling chronic pain sufferers to live fuller lives.
Segmentation Analysis:
The market’s segmentation by Treatment Type highlights the enduring relevance of the pharmacologic segment. This includes a range of medications from anti-depressants to muscle relaxants and, most prominently, anticonvulsants. The continued reliance on these drugs, despite their limitations, underscores the urgent need for novel, more efficacious, and safer alternatives. The emerging digital therapeutic segment, though smaller, is set to disrupt the market by offering scalable, personalized, and convenient non-pharmacological interventions. These digital tools are particularly appealing to younger demographics and those seeking alternatives to lifelong medication.
By Pharmacologic Class, anticonvulsants / α2δ ligands continue to lead the market. These drugs work by modulating neurotransmitter activity, reducing the hyperexcitability of nerve cells responsible for pain signaling. While effective for many, their side effect profiles necessitate continuous innovation. Analgesics / NSAIDs form another crucial, albeit often adjunctive, pharmacologic class, primarily used for acute pain management rather than foundational fibromyalgia treatment. The focus of future R&D is increasingly on developing drugs with novel mechanisms of action that offer superior efficacy and fewer adverse effects.
Fibromyalgia Treatment Market Top Companies

- Pfizer
- Eli Lilly
- Allergan / AbbVie
- Teva Pharmaceutical Industries
- Novartis
- Sun Pharmaceutical Industries
- Astellas Pharma
- Tonix Pharmaceuticals
- Aptinyx
- Axsome Therapeutics
- Scilex
- Johnson & Johnson
- Medtronic
- Boston Scientific
- Nevro Corp.
- Abbott
Latest Announcements by Industry Leaders
- In July 2024, the FDA granted fast-track designation to a sublingual tablet form of the muscle relaxant cyclobenzaprine hydrochloride for the management of fibromyalgia. Tonix Pharmaceuticals reported that its patented form of the drug, Tonmya, received the designation following the results of the phase 3 RESILIENT study. In that study, Tonmya significantly reduced daily pain compared with placebo in patients with fibromyalgia.
Recent Developments
- In October 2024, Lin Health, a digital platform helping people reclaim their lives from chronic pain, announced a collaboration with Mayo Clinic to improve care transition support for patients who complete the Fibromyalgia Treatment Program on Mayo Clinic’s campus in Rochester, Minnesota. Following the initial few months of results, the collaboration is slated to expand to support Mayo Clinic primary care patients in Arizona, providing an at-home, high-touch digital intervention that offers patients longitudinal, real-time support for chronic pain.
- In January 2025, Silo Pharma, Inc., a developmental-stage biopharmaceutical company focused on developing novel formulations and drug delivery systems for traditional therapeutics and psychedelic treatments, announced that the U.S. Patent and Trademark Office (USPTO) issued a notice of allowance for patent application 17/954,864 for pharmacological prophylactics against stress-induced affective disorders in females.
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344